EndoTrap® FAQs
FAQs
Frequently Asked Questions (FAQs)
What is EndoTrap®?
EndoTrap® is an affinity chromatography resin based on a bacteriophage-derived protein which binds to endotoxins with high affinity and specificity. The EndoTrap® protein captures the conserved region of the inner core of LPS molecules and is thereby able to bind to all kind of endotoxins from Gram-negative bacteria. It is neither an antibody, a synthetic peptide nor Polymyxin B-based.
What is the principle of EndoTrap®?
EndoTrap® is an affinity matrix system. EndoTrap® can be used in either batch or chromatography mode.
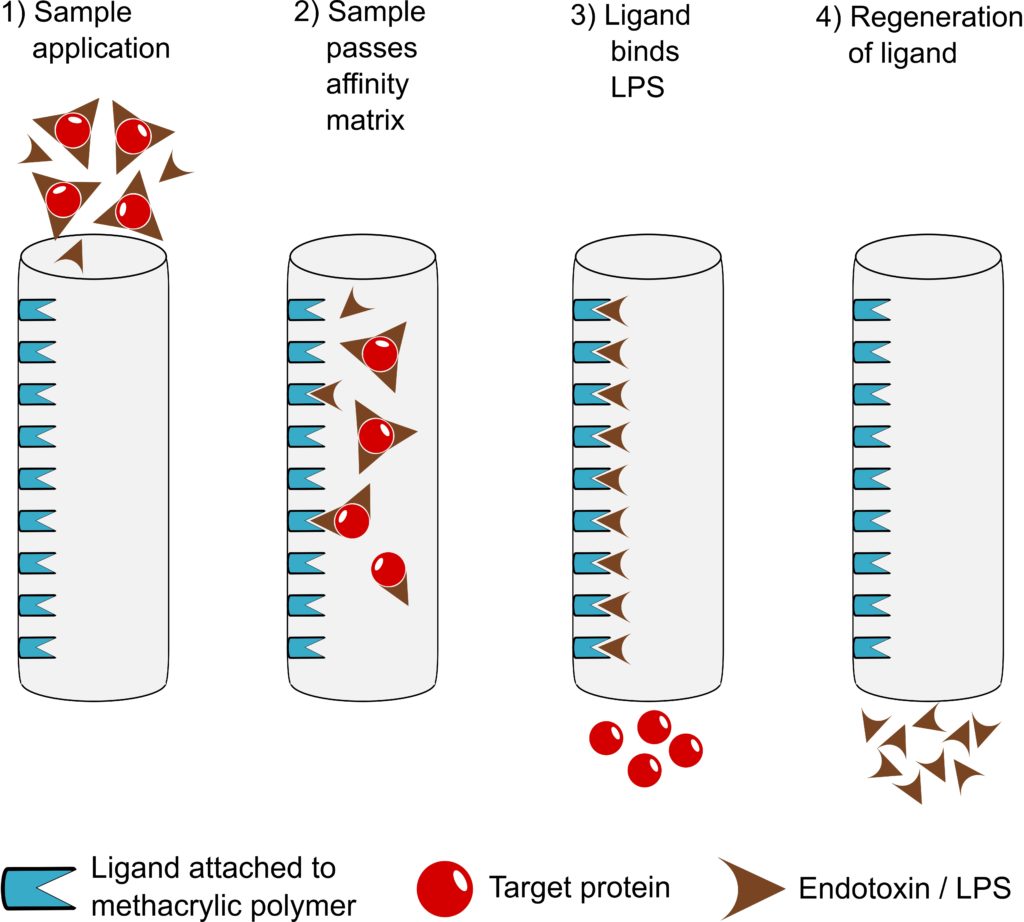
What are the main advantages of EndoTrap® in comparison to other products?
EndoTrap® is very endotoxin-specific, leading to exceptionally high sample recovery and endotoxin removal rate at the same time. Accordingly, it shows a very stable performance at a wide variety of conditions (pH and ionic strength/salt concentration). Furthermore, it is an easy-to-use flow-through system and non-toxic (in contrast to Polymyxin B). For regulatory purposes, toxicological data, a Regulatory Support File and a Leakage ELISA (EndoTrap® HD) are available.
How can I use EndoTrap®?
EndoTrap® can be used either in column or batch mode. Generally, column mode is easier to handle and more efficient in comparison to batch mode. Batch mode may be applied for small volumes and increased contact time. However, parameters such as pH, ionic strength, temperature, contact time, etc. may have to be optimized for each application to obtain maximum endotoxin removal with minimum loss of product.
What is batch mode?
Chromatography is traditionally made in two modes: batch (or discontinuous) and continuous (column mode) chromatography. For batch endotoxin removal, the settled gel is simply added directly to the sample solution. Several contact times ranging from 3 to 20 min should be tested to determine the best endotoxin removal rate.
What is column mode?
Columns are easily prepared by packing with the depyrogenated EndoTrap® resin as 50% slurry in sterile buffer.
How to work with a real flow through system?
EndoTrap® is a real flow through system (“ready-to-use” columns). You can apply your sample (up to 50 ml) continuously to a 1 ml column – up to 3 ml at once. If desired you can additionally purchase a funnel, which enlarges the loading volume to 20-25 ml. We recommend regenerating the column after each cleaning step. One cleaning step means that your complete working volume passed through the column once. Afterwards you can fill in your sample volume again.
Is it possible to use a peristaltic pump instead of gravity flow?
Yes, but do not exceed a speed of 1 ml/min. The slower the speed, the more efficient is the endotoxin removal. Gravity flow guarantees a flow rate of about 0.5 ml/min. Higher flow rates are possible with EndoTrap® HD. Make sure that all equipment is completely endotoxin free!
Is EndoTrap compatible with fully automated liquid chromatography systems?
Yes it is. You can purchase pre-packed chromatography columns from LIONEX (please see our catalogue). You can also pack your own columns but you must have experience and equipments for packing proper columns using EndoTrap® resin in endotoxin free liquid chromatography columns. Ensure that the run time does not exceed 1 ml/min, otherwise the gel bed will be compressed (EndoTrap® red). With EndoTrap® HD higher flow rates are possible.
Can EndoTrap® be regenerated?
Yes, with the provided regeneration buffer. EndoTrap® red can be reused at least 3 times, EndoTrap®HD up to 10 times, without loss of endotoxin removal efficiency. Store EndoTrap® in regeneration buffer enriched with 0.02% sodium azide to avoid growth of bacteria.
How high is the binding capacity of EndoTrap®?
EndoTrap® red: 2 million endotoxin binding sites per ml (1 EU = 100 pg LPS)
EndoTrap® HD: 5 million endotoxin binding sites per ml (1 EU = 100 pg LPS)
How to calculate the number of required cleaning steps (EndoTrap® red)?
In case your initial endotoxin concentration is very high or you wish to reach a very low concentration, EndoTrap® can be applied in a consecutive manner several times (EndoTrap®red at least three times, EndoTrap® HD up to ten times). Each round of application theoretically yields a two log reduction of endotoxin (three log reduction with EndoTrap® HD). However, parameters such as pH, ionic strength, temperature, contact time, etc. may have to be optimized for each application to obtain maximum endotoxin removal with minimum loss of product. Furthermore, the performance may vary with the sample properties, especially of proteins. Depending on your LPS starting concentration [EU] you must perform a certain number of cleaning steps in order to achieve your desired LPS end concentration [EU]. To achieve best results and to be able to calculate which package size you need for your desired performance, total LPS units applied should not exceed 30-50% of the maximum resin capacity.
LPS removal from buffers: With repetitive use of EndoTrap®, you can achieve concentrations as low as 0.005 EU/ml (limit of endotoxin detection). LPS removal from proteins: With repetitive use of EndoTrap®, decrease to concentrations as low as 0.1 EU/ml is possible. As it is a biological system, the efficiency of EndoTrap® slightly decreases at low endotoxin contamination levels. At 0.1 EU/ml the removal efficiency is approximately 70% for each following purification step.
LPS removal from DNA:With repetitive use of EndoTrap®, you can reach 0.01 EU/ml.
If you do not obtain your desired endotoxin level after the third cleaning step (each cleaning step ends with a regeneration step), please contact us and take advantage of LIONEX technical support!
When does the sample (proteins, peptides, antibodies, plasmid DNA) elute?
The void volume of 1-ml-EndoTrap®-column is 0.3-0.5 ml. Commonly, the sample will elute immediately after this volume has passed through the column. In case there is a slight interaction between sample and EndoTrap®, elution is delayed. Washing with equilibration buffer and collecting fractions is recommended under these circumstances. Ultimately, fractions containing your target material can be pooled.
What can I do if the flow through rate is slow?
Gravity flow usually leads to a flow rate of about 0.5 ml/min. A solution of 10 ml thus needs 20 minutes to pass through a 1-ml-column. In case of a much lower flow rate, please check for bubbles in the column which may emerge during transport. There are several ways for removing bubbles from EndoTrap® columns. One way is centrifugation of the closed column at ~1000 x g for 5 minutes. Alternatively, you may slightly press the frit on the top level with a pyrogen-free pipette. Another possibility for removing bubbles is connecting a 5-ml syringe to the bottom of the column via a flexible, endotoxin-free tube. Make sure that the column is open and that there is buffer in the column. Use the syringe to aspirate bubbles.
Can EndoTrap® be used at 4 °C?
Yes, EndoTrap® is fully functional at 4 °C as well as at room temperature.
Is it possible to use EndoTrap® combined with chaotropic substances e.g. urea?
Yes, at pH 7 up to 2 M urea can be used without any significant decrease of endotoxin removal efficiency.
Are there inhibitory agents known for EndoTrap®?
EndoTrap® HD is inhibited by EDTA and other calcium chelators such as EGTA, HEDTA, NTA, Citrate,
Ammoniumsulphate, SDS and other detergents. EndoTrap® red is inhibited by salt concentrations above 250 mM, GdnHCl, Ammoniumsulphate, SDS and other detergents.
Do reducing agents such as Dithiothreitol (DTT) disturb the endotoxin removal of EndoTrap®?
EndoTrap® red is disturbed, but EndoTrap® HD can be used with DTT (up to 10 mM) or be pre-washed with DTT before use.
Is it possible to use EndoTrap® at high ionic strength?
EndoTrap® HD can be used with up to 1 M NaCl without significant decrease of endotoxin removal efficiency. EndoTrap® red is not appropriate for high ionic strength. It can only be used up to 250 mM NaCl (best results at <100 mM NaCl).
Can the EndoTrap® material be autoclaved, sanitized or otherwise sterilized?
EndoTrap® should not be autoclaved, but you can wash the resin with 30% Ethanol. Avoid sanitation with sodium hydroxide, since it may denature the ligand.
Which types of substances and in which concentration / volume can I apply to the EndoTrap®
column?
In general, every type of substance can be applied onto the column, as long as it can pass through the column. There is no limit concerning the molecular weight of proteins or substances such as proteins, peptides or antibodies. EndoTrap® removes endotoxin from proteins with isoelectric points (pI) from 5 to 9. EndoTrap® HD also works with DNA (tested for plasmid DNA and RNA) and plant extracts. Protein concentrations higher than 50 mg/ml have successfully been applied onto the system. However, we recommend a work concentration of 1-10 mg/ml. 1 to 10 column volumes are usually ideal. Up to 50 ml can be applied onto a 1 ml EndoTrap® column without loss of endotoxin removal efficiency (1 column volume “ready-to-use” = 1 ml). For EndoTrap®, HD we recommend a maximum LPS load of 2.5 million EU/ml resin.
Does the EndoTrap® system bind all pyrogens?
The class of pyrogens consists of manifold molecules and polymers with stimulating activity. However, the vast majority comes from bacterial endotoxins. EndoTrap® binds to a conserved region of the inner core of the lipopolysaccharide (LPS) molecules and can thereby bind to all kinds of endotoxins from Gram-negative bacteria.
Does the EndoTrap® system bind yeast pyrogens?
The yeast cell wall is completely different from the outer membrane of Gram-negative bacteria and does not contain endotoxins. Nevertheless, in numerous cases, it makes sense to clean yeast extracts with EndoTrap® because bacterial species occuring in laboratory water supplies are common sources for endotoxin contamination during downstream processes. LPS from most of these water contaminants is efficiently removed by EndoTrap®.
What do I have to consider while working with proteases?
Proteases may destroy the EndoTrap® ligand during LPS removal. Be sure to perform the cleaning steps at conditions where your protease is less active, e.g. at 4°C, or change the buffer composition if possible. For instance, the protease pepsin does not work at pH above 6, thus favouring this condition for endotoxin removal with EndoTrap®.
Lionex GmbH
Lionex Diagnostics and Therapeutics GmbH
Salzdahlumer Straße 196, 38126 Braunschweig, Germany
Lionex
© 2018 Lionex GmbH
Last update: Lionex GmbH
LIONEX GmbH
